Pathophysiology of Dystonia
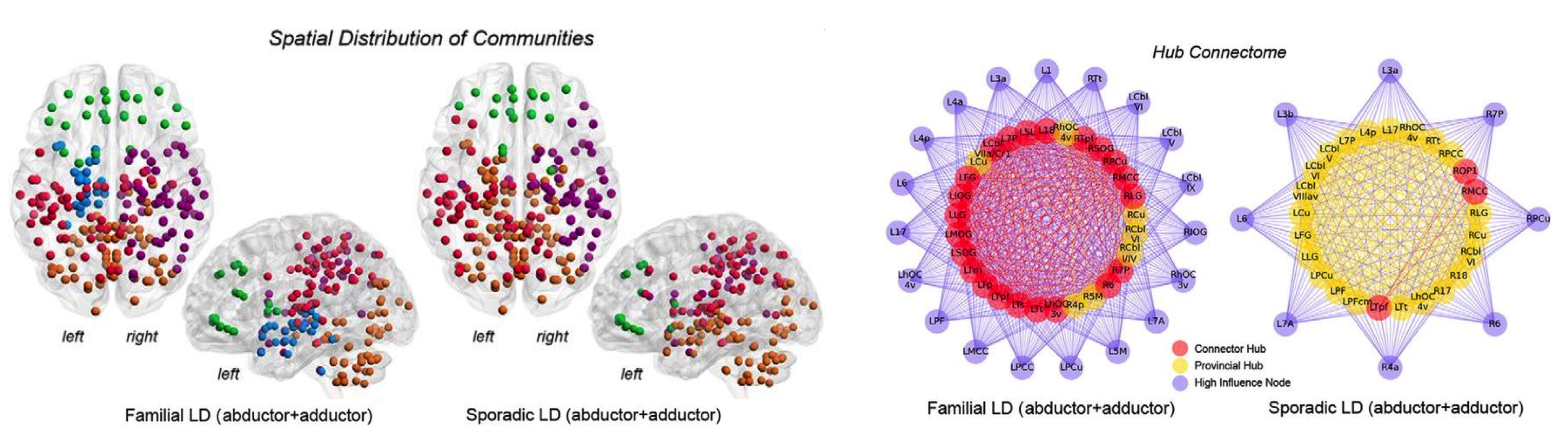 Functional architecture of neural networks in patients with familial and sporadic forms of laryngeal dystonia (i.e., spasmodic dysphonia). Left panel: spatial topology of functional communities in the group-averaged networks. Right panel: distribution of bivariate provincial (yellow) and connector (red) hubs and their connectivity profiles with high-influence nodes (purple) in familial and sporadic laryngeal dystonia (Fuertinger S and Simonyan K, J Neurosci, 2017).
Functional architecture of neural networks in patients with familial and sporadic forms of laryngeal dystonia (i.e., spasmodic dysphonia). Left panel: spatial topology of functional communities in the group-averaged networks. Right panel: distribution of bivariate provincial (yellow) and connector (red) hubs and their connectivity profiles with high-influence nodes (purple) in familial and sporadic laryngeal dystonia (Fuertinger S and Simonyan K, J Neurosci, 2017).
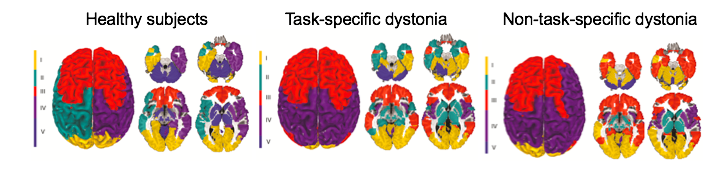
Large-scale neural community architecture during the resting state in healthy subjects and its pathophysiological disorganization in patients with task-specific dystonia (laryngeal dystonia and writer's cramp) and patients with nontask-specific dystonia (cervical dystonia and blepharospasm) (Battistella G et al. Cerebral Cortex, 2017).
Task-specific focal dystonias are characterized by selective activation of dystonic movements during the performance of highly learned motor tasks, such as writing or speaking. To date, we have only limited knowledge about the distinct neural abnormalities that lead to the development of task-specificity in focal dystonias, which affect similar muscle groups but result in different clinical manifestations, such as writer’s cramp vs. pianist’s dystonia or spasmodic dysphonia vs. singer's dystonia. Funded by the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NINDS/NIH R01NS088160), our goal is to dissect the pathophysiological mechanisms underlying the phenomenon of task specificity in isolated focal dystonias using multi-level brain network analysis in conjunction with neuropathological examination of postmortem brain tissue from patients with dystonia. Rather than viewing these disorders as interesting curiosities, understanding the biology of task-specific activation of motor programs is central to understanding dystonia.
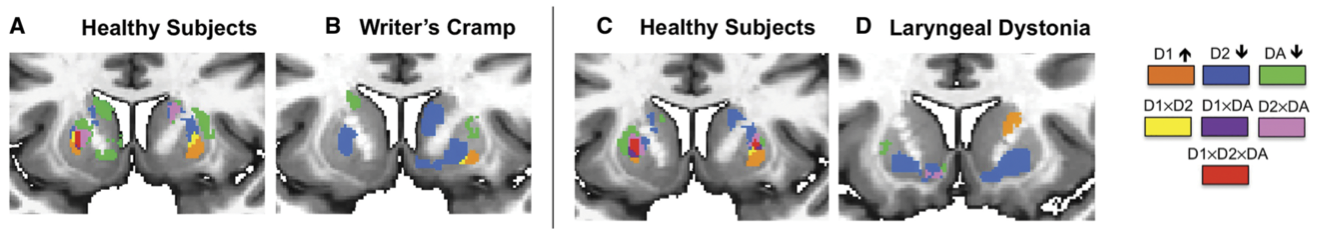
Topological distribution of striatal dopaminergic function in healthy subjects and patients with writer’s cramp and laryngeal dystonia (Simonyan K et al., Brain 2017).
Despite the recent progress in elucidating functional brain abnormalities within the basal ganglia-thalamo-cortical circuitry in focal dystonias, there is a fundamental gap in understanding the neurochemical correlates underpinning the functional alterations in these disorders. Our goal is to provide detailed knowledge about the neurotransmission via GABAA, D1- and D2-family receptors in patients with different forms of focal dystonia. This information will help determine the contribution of GABAergic and dopaminergic neurotransmission to the pathophysiology of dystonia, as well as identify potential new pharmacological targets for novel treatment options.

 Dystonia and Speech Motor Control Laboratory | Department of Otolaryngology-Head & Neck Surgery, Massachusetts Eye and Ear and Harvard Medical School | 243 Charles Street, Suite 421 | Boston, MA 02114 |
Dystonia and Speech Motor Control Laboratory | Department of Otolaryngology-Head & Neck Surgery, Massachusetts Eye and Ear and Harvard Medical School | 243 Charles Street, Suite 421 | Boston, MA 02114 |